ABOUT SECANT GROUP
As part of the Solesis family of companies, Secant Group and Polyzen work closely together to integrate advanced textiles, polymers, and hybrids into medical device and delivery system solutions. Through integrated research and development activities, we are helping to bring new technologies and therapies to market.
About Secant Group, LLC specializes in the design and development of advanced, custom-engineered textiles and biomaterial solutions that support the body’s natural ability to repair, recover, and regenerate. With decades of expertise in the development and manufacturing of implantable medical components, Secant empowers innovators in medical devices and drug delivery to push boundaries and bring transformative technologies to patients in need of better options. From initial prototyping to full-scale commercialization, Secant is the trusted partner for integrated, collaborative development across cardiovascular, neurovascular, orthopedic, surgical, and pharmaceutical applications.
About Polyzen is a leading developer and manufacturer of customized polymer-based materials, films, components, and assemblies for the medical device industry. Our range of material and processing technologies allow us to provide optimum solutions for innovation-driven companies, ranging from entrepreneurial start-ups to major medical device manufacturers. From prototype development through full-scale production, we take pride in our quality, technical expertise, and customer service, and we strive to provide the optimal polymer-based solution for our customers throughout the product lifecycle.
Together, Secant Group and Polyzen deliver a synergistic approach to breakthrough medical innovation.
About Solesis
Solesis integrates biomaterial solutions for the MedTech and Biopharma industries. Through integrated research, development, and manufacturing capabilities, the Solesis family of companies—Charter Medical, Polyzen, SanaVita Medical, and Secant Group—enable technologies and therapies for next-generation patient care.
FEATURED ARTICLES
-
Ideally, device engineers should partner with a textile component supplier as soon as possible during the earliest stages of product development. But how can a device manufacturer pinpoint the right textile partner?
-
Different considerations often apply to raw material selection, prototyping, and scale-up, and medical device manufacturers can be at a loss for where to start.
-
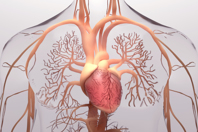
New technologies and fabric modifications must be developed to meet both well-understood and emerging needs in the cardiovascular device space.
-
Biomedical textile engineering affords critical innovations in scaffold design, particularly where structural repeatability, spatial geometry, and engineering strength support the cellular processes in a consistent fashion. These factors illustrate the important impact that the convergence of a traditional installed base of engineering techniques–combined with new materials—offer for successful advances in regenerative medicine.
-
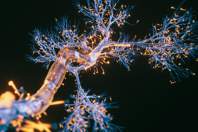
In the neurovascular device segment, development is focused on minute, precision devices delivered via micro-catheters. The overarching challenge is to create components that maximize performance within a minimal deliverable profile. That’s where biomedical textiles come in.
-
Medical device manufacturers are expanding their portfolios with biologically based products for the repair of musculoskeletal defects ranging from bone grafting and fusion, motion-preserving spinal repair, to cartilage repair and ligament and tendon tears.
-
Learn how fabrics can be used in heart valves now and in the future, and explore design considerations during product development.
-
In this case study, Secant Group creates braided and woven fabrics for a textile-based component in a minimally invasive orthopedic device.
-
Secant Group’s textile braiding and engineering expertise accelerates prototyping process for new neurovascular device in this case study.
-
Medical textile supplier successfully reverse-engineers a woven textile component based on the original sample to help medical equipment manufacturer avoid sales disruption.
-
We review what items a device manufacturer and supplier should align on and what essential assets a manufacturer should look for in a supplier team.
-
This article discusses best practices on how medical component suppliers can mitigate risks for critical raw materials, change management, operational consistency, and manufacturing assets.
CONTACT INFORMATION
Secant Group & Polyzen
1041 Classic Rd.
Apex, NC 27539
UNITED STATES
Phone: 919-319-9599